The next installment will contain a little more quantum physics, as it is necessary for understanding orbitals and bonds. Bonds are very important in lipid chemistry. Anyone into Nourishing traditions and similar lifestyles knows that you can't cook with saturated fats due to the double bonds. These are best explained with a little quantum physics basics.
Today we are just going to go over some of the basics of organic chemistry. We'll start with the atom, and today's lesson will end with alkanes.
Lets get started, shall we?
Everything in the chemical world is made up of three things; Protons, Neutron, and Electrons.
Now, Protons have a positive charge and are usually represented in print as 'p+'
Neutrons have a negative charge and are represented as 'n', sometimes with a little superscript zero to show that they have no charge.
Finally, electrons have a negative charge and a represented as 'e-'
"So what Zeke?" you're saying to me, "I already know this, I went to middle school."
Just bear with me braniac.
Protons and neutrons are about the same size as each other. They both have a mass of one Atomic Mass Unit (AMU). Electrons on the other hand are very tiny. They are so tiny in fact, that even though they do have a mass, it is considered to be nothing. Only hardcore particle physicists actually care about the mass of electrons.
Protons, neutrons, and electrons mix together to form atoms. Protons and neutrons lie at the center of an atom in the nucleus. Electrons fly around the outside in orbits. The simplest Atom is Hydrogen. It has one proton in the nucleus and one electron orbiting it, and It chemical symbol is 'H'. What makes one element different from another is the number of protons. Hydrogen has one, carbon has six, and uranium has 92.
This is where things get tricky.
Here is the traditional solar system orbital model you probably learned in school.
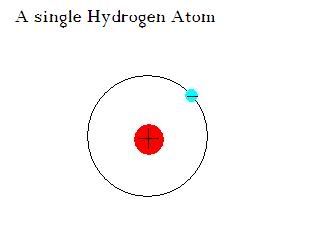
The solar system type model is a LIE!
In actuality electrons don't really 'move' around the nucleus. They kinda pop out of existence in their current location, while simultaneously popping back into existence someplace else. Its weird, I know.
It gets weirder. These 'orbitals' aren't anything like the orbit of a planet. They are just locations around the nucleus where there will be a good chance of finding an electron. Electron 'orbits' are just a statistic. This creates what is known as the 'electron cloud theory.' This makes our hydrogen atom actually look more like this:
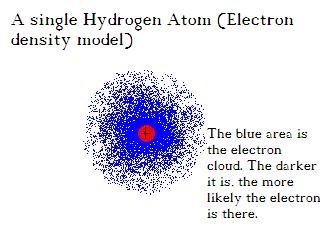
I told you it was weird. Instead of having a nice neat orbital, we have a area of 'electron density. I like to visulaize it as a could of gas, or a blob of gelatinous stuff surrounding the nucleus.
The thing is, that kind of model isn't always helpful to chemists, because well... we're chemists, not physicists. When we're being chemists and doing our chemist thing, we usually write the molecule like this:
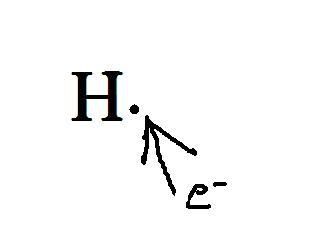
This is just the symbol for the element, with a dot for the electron. When doing this we only show the electrons for the outermost orbital, since these are the only electrons that bond. This is simple for hydrogen since it only has one orbital that can be filled with a maximum of two electrons.
If you've never taken any type of college level chemistry course, your brain is probably tired. If it is, go get a glass of water, give your brain a minute to relax, and come on back. Things are going to be simpler for the rest of the lesson.
Welcome back (if you left). Let's continue our discussion of electrons. Electrons have some issues. They're awfully codependent. They can't stand to be alone. Electrons always occur in pairs. Its their nature. So what about or hydrogen from before? He only had one electron.
Well, hydrogen doesn't occur like that in nature. It always pairs up with another hydrogen, and they share electrons. Here's the picture:

Since two elements are coming together, this is no longer an atom. It is now a molecule.
The outermost electron orbital (or more commonly, 'shell') s known as the 'valence shell' and the electrons within it are 'valence electron.' Elements like to have their valence shells filled as well as having electron pairs in them. Most electrons like to have 8 electrons in their outer shell, the only exception we will concern ourselves with is hydrogen, which only needs two. Go back and look at that hydrogen molecule, It's stable and happy. Can't you see how happy it is? No? Look closer.
The hydrogen molecule is sharing a valence electron with the other, so both of them have full valence shells at the same time. This is called a covalent bond.
The vast majority of bonds in organic chemistry are covalent. We'll talk about Ionic bonds tomorrow.
On to CARBON!
Organic chemistry was originally the study of chemicals produced by lifeforms. Since all known life is carbon based, these were carbon based compounds. Now days organic chemistry is the chemistry of all carbon compounds, whether they are organic or not.
Let's meet our friend Carbon, shall we?
Carbon has six protons and six electrons. Only four of these electrons are valence electrons. They are lonely and have no one to love. Here's a picture of carbon:
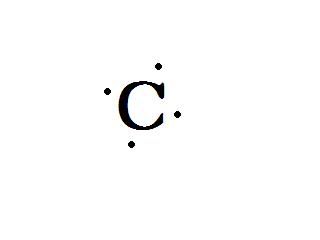
Man he looks so sad. unpaired electrons and an empty valence shell. I know! Lets send in some hydrogens! They only need one more electron each. Four should do it.
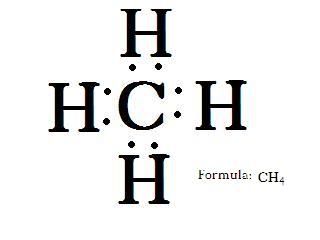
Look at that! We just made methane. Methane is the simplest organic compound. It's also the simplest hydrocarbon. Its called a hydrocarbon because, you guess it, its made of HYDROgen and CARBON. It is also the simplest alkane. Alkanes are the simplest type of hydrocarbon. Alkanes are what we call saturated hydrocarbons, they have no double or triple bonds. More on that later.
Carbon is a very special atom. Not only can it covalently link with other atoms like hydrogen, it can covalently link with another carbon. So we'll take two carbons and link them, then pair any unpaired electrons with hydrogen. Notice that carbon ALWAYS forms four bonds. This will become very important later.
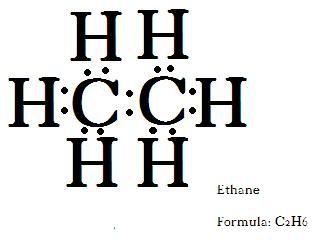
This is ethane. It is the next largest alkane. Notice how they both end in the -ane suffix? That is how we know its an alkane. If we added another carbon, we'd have the next smallest alkane. The prefix lets us know how many carbons there are. It is important to know the names of the alkanes, because the names of all other organic compounds are derived from them. Here is a list. learn it, memorize it, love it with all your heart:
The number is the number of carbons
1. Methane
2. Ethane
3. Propane
4. Butane
5. Pentane
6. Hexane
7. Heptane
8. Octane
9. Nonane
10. Decane
11. Undecane
12. Dodecane
13. Tridecane
14. Tetradecane
15. Pentadecane
16. Hexadecane
17. Heptadecane
18. Octadecane
19. Nonadecane
20. Eicosane
The 'normal' form of these compounds is in a straight chain. These are called straight chain hydrocarbons and usually have an italics 'n' in front of then because they are 'normal.' i.e. n-pentane, n-nonane, n-eicosane
Now chemists are lazy. Drawing all those little dots for electron is a lot of work, so we usually just draw a single line. Here are some examples.
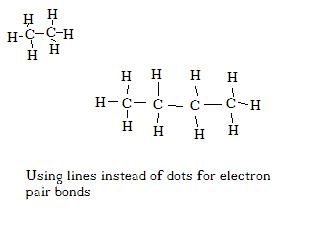
Of course for big chemicals that can be a lot of work too. So we use something called 'organic shorthand'. We just draw the lines, and ignore the symbol for the carbon, and ignore the hydrogens completely. In organic chemistry they are a given.
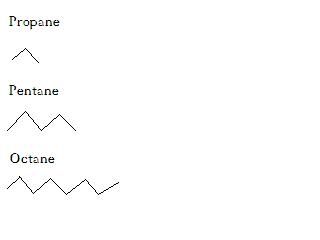
OF course more than one carbon can bond to another carbon. These are called branched hydrocarbons. I'm not going to go into how they are named, but here are a few in organic shorthand. They can even bend back on themselves and make loops! While this is not of too much import for our lipid discussion, if you want to get deeper into organic chemistry it is very important to things like polysaccharides.
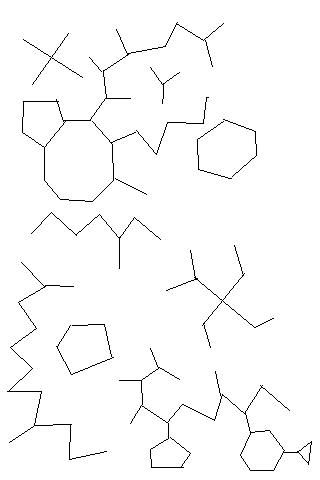
As you can see, the possibilities are virtually limitless!
That pretty much concludes today's post. Its alot to digest as it is and I actually wanted to go into more detail. If you have questions about anything, please feel free to ask it in a comment.
Tomorrow we will talk about ions, and go into compounds that incorporate oxygen into their structure.

This was a very fun post to read. Especially for someone who missed out on both chemistry AND physics in highschool, (I know, how lame huh?) only to find out later on in her twenties that she LOVES science, especially the kind that can bring clarity to the fine print of nutrition. I'm looking forward to reading the rest of this series. Keep up the good work!
ReplyDelete